IOVS (2022)
Yannik Laich, Julian Wolf, Rozina Ida Hajdu, Anja Schlecht, Felicitas Bucher, Laurenz Pauleikhoff, Martin Busch, Gottfried Martin, Henrik Faatz, Saskia Killmer, Bertram Bengsch, Andreas Stahl, Albrecht Lommatzsch, Günther Schlunck, Hansjürgen Agostini, Stefaniya Boneva, Clemens Lange
Number of citations (crossref.org): Loading....

Purpose: Proliferative vitreoretinopathy (PVR) remains an unresolved clinical
challenge and can lead to frequent revision surgery and blindness vision loss. The
aim of this study was to characterize the microenvironment of epiretinal PVR tissue,
in order to shed more light on the complex pathophysiology and to unravel new treatment options.
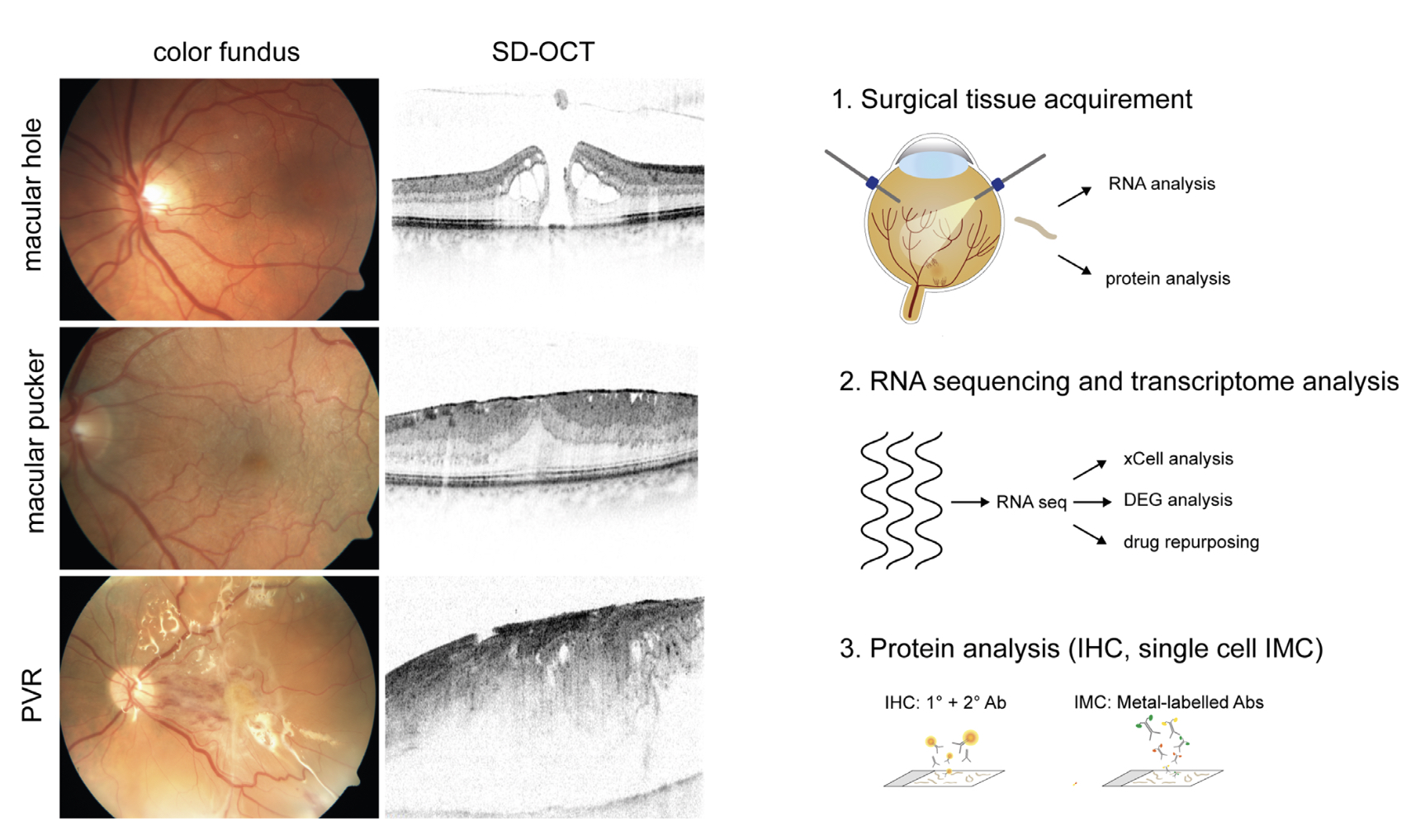
Methods: A total of 44 tissue samples were analyzed in this study, including 19 epiretinal PVRs,
13 epiretinal membranes (ERMs) from patients with macular pucker, as well as 12 internal limiting
membranes (ILMs). The cellular and molecular microenvironment was assessed by cell type deconvolution
analysis (xCell), RNA sequencing data and single-cell imaging mass cytometry. Candidate drugs for PVR
treatment were identified in silico via a transcriptome-based drug-repurposing approach.
Results: RNA sequencing of tissue samples demonstrated distinct transcriptional profiles of PVR, ERM,
and ILM samples. Differential gene expression analysis revealed 3194 upregulated genes in PVR compared
with ILM, including FN1 and SPARC, which contribute to biological processes, such as extracellular matrix
(ECM) organization. The xCell and IMC analyses showed that PVR membranes were composed of macrophages,
retinal pigment epithelium, and α-SMA-positive myofibroblasts, the latter predominantly characterized by
the co-expression of immune cell signature markers. Finally, 13 drugs were identified as potential
therapeutics for PVR, including aminocaproic acid and various topoisomerase-2A inhibitors.
Conclusions: Epiretinal PVR membranes exhibit a unique and complex transcriptional and cellular profile
dominated by immune cells and myofibroblasts, as well as a variety of ECM components. Our findings provide
new insights into the pathophysiology of PVR and suggest potential targeted therapeutic options.