IOVS (2023)
Laurenz Pauleikhoff, Stefaniya Boneva, Myriam Boeck, Anja Schlecht, Günther Schlunck, Hansjürgen Agostini, Clemens Lange, Julian Wolf
Number of citations (crossref.org): Loading....

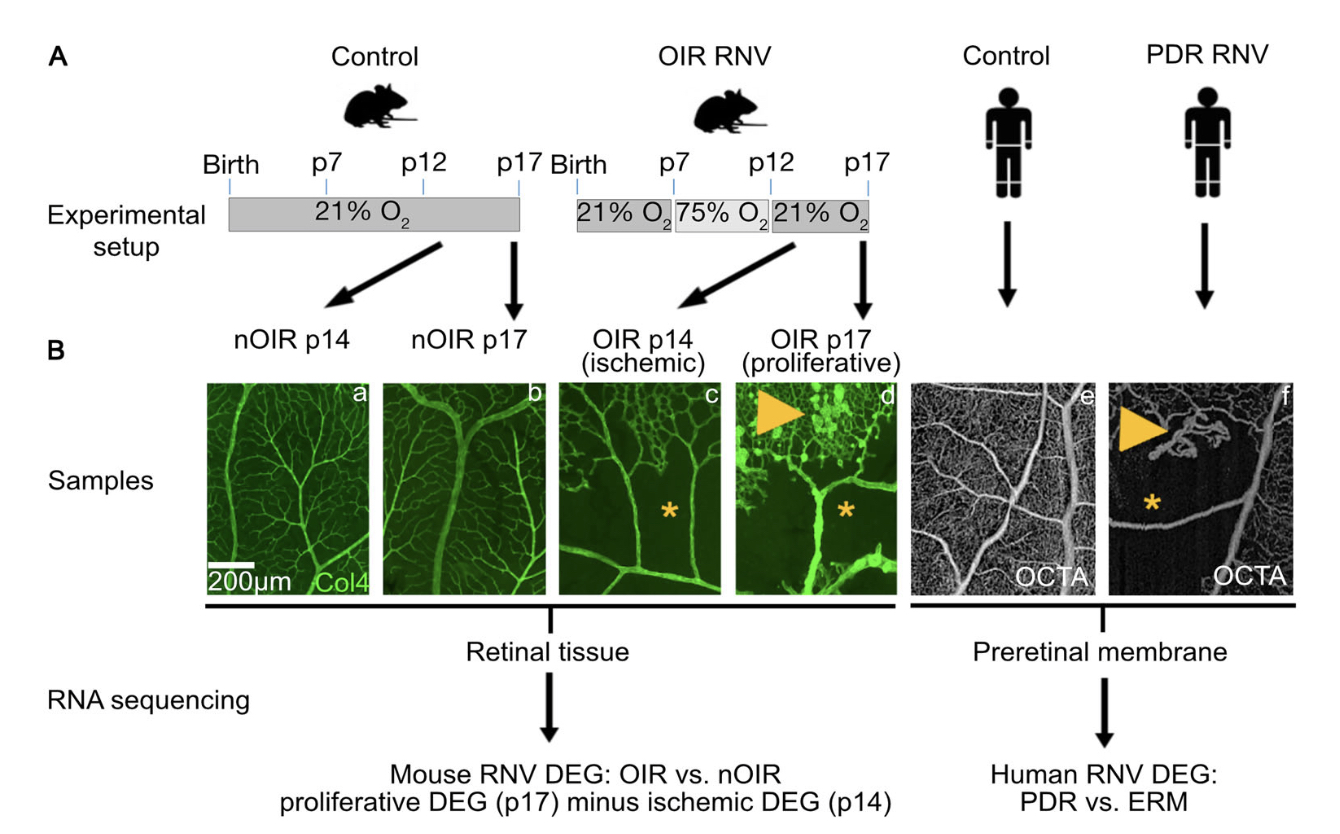
Purpose: Retinal neovascularization (RNV) is the leading cause of vision loss in diseases
like proliferative diabetic retinopathy (PDR). A significant failure rate of current treatments
indicates the need for novel treatment targets. Animal models are crucial in this process, but
current diabetic retinopathy models do not develop RNV. Although the nondiabetic oxygen-induced
retinopathy (OIR) mouse model is used to study RNV development, it is largely unknown how closely
it resembles human PDR.
Methods: We therefore performed RNA sequencing on murine (C57BL/6J) OIR retinas (n = 14)
and human PDR RNV membranes (n = 7) extracted during vitrectomy, each with reference to
control tissue (n=13/10). Differentially expressed genes (DEG) and associated biological
processes were analyzed and compared between human and murine RNV to assess molecular overlap
and identify phylogenetically conserved factors.
Results: In total, 213 murine- and 1223 human-specific factors were upregulated with a small overlap
of 94 DEG (7% of human DEG), although similar biological processes such as angiogenesis, regulation of
immune response, and extracellular matrix organization were activated in both species. Phylogenetically
conserved mediators included ANGPT2, S100A8, MCAM, EDNRA, and CCR7.
Conclusions: Even though few individual genes were upregulated simultaneously in both species,
similar biological processes appeared to be activated. These findings demonstrate the potential
and limitations of the OIR model to study human PDR and identify phylogenetically conserved potential treatment targets for PDR.